2024
Presently (Q3 2024), six Novel Food authorisations have entered into force and there are eight positive EFSA opinions on edible insects. Find out more below.
On 29 July 2024, EFSA published its 8th positive opinion for edible insects as Novel Food, on house cricket powder (Acheta domesticus).
On 1 June 2023, EFSA published its 7th positive opinion for edible insects as Novel Food, corresponding to the one on UV-treated powder of whole yellow mealworm (Tenebrio monitor).
The respective European Commission Implementing Regulations authorising their commercialisation are expected to be published soon.
2023
Following EFSA’s 5th opinion on edible insects from May 2022, in 2023 the European insect sector also welcomed the Commission’s Implementing Regulation authorising partially defatted whole house cricket (Acheta domesticus) as Novel food. The authorisation came into effect on 24 January 2023.
Read more about it in the IPIFF Press Statement.
INFO SHEET on the commercialisation of edible insects in the EU
INFO SHEET – Types of Novel Food Applications
2022
At the beginning of 2022, the European Commission published the second Implementing Regulation for frozen, dried and powder yellow mealworm (Tenebrio Molitor), and the first authorisation of dried, powder and frozen house cricket (Acheta domesticus), following the 3rd and 4th EFSA opinions from August 2021. The authorisations entered into force respectively on 1 and 3 March 2022 Read more about it in the IPIFF Press Statement.
In May 2022, the Parma-based Agency (EFSA) published the 5th opinion on edible insects, namely on the partially defatted house cricket (Acheta domesticus).
On 4 July 2022, EFSA published its 6th opinion on edible insects, namely on the safety of frozen and freeze-dried formulations of the lesser mealworm (Alphitobius diaperinus larva). This authorisation is the second for this species and follows the EFSA opinion published in May 2022.
2021
Following the 1st EFSA opinion covering an insect species (January 2021), dried yellow mealworm (Tenebrio molitor) has been authorised at the EU level as the first insect food product following the enter into force, on 22 June 2021, of the Commission Implementing Regulation authorising its commercialisation. entered into force in June 2021 (more in the IPIFF Press Release).
Following the 2nd positive opinion (July 2021), the European Commission authorised dried and frozen migratory locust (Locusta migratoria) following the entering into force of the Commission Implementing Regulation authorising its commercialisation at the EU level, which entered into force on 3 December 2021. For more information, please refer to the IPIFF Press Statement.
A list of Frequently Asked Questions (FAQs) – 2023 version – on this subject may be accessed here. These FAQs are also covered by the IPIFF Briefing paper on novel foods (updated in 2021).


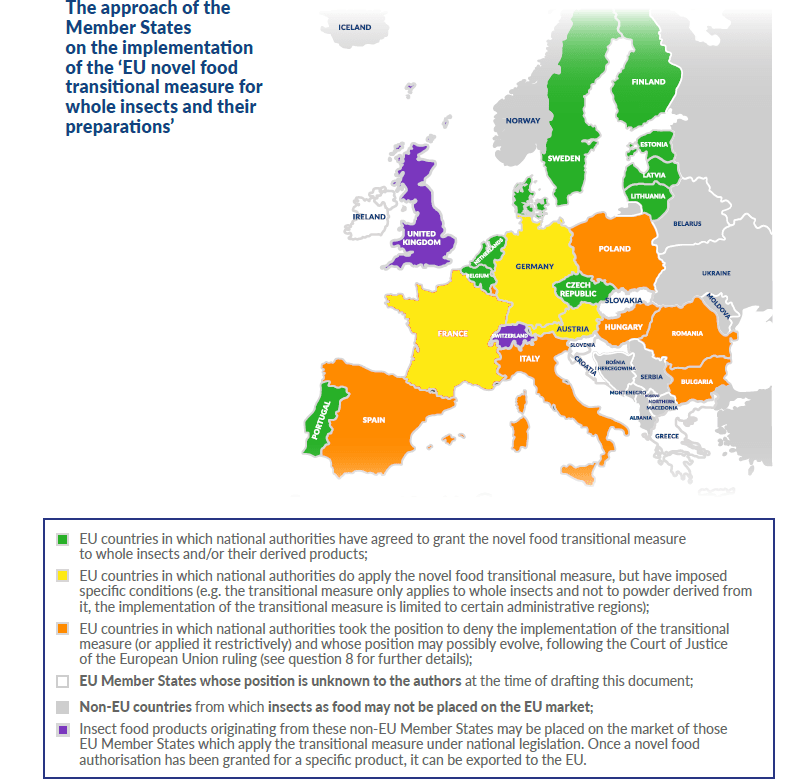

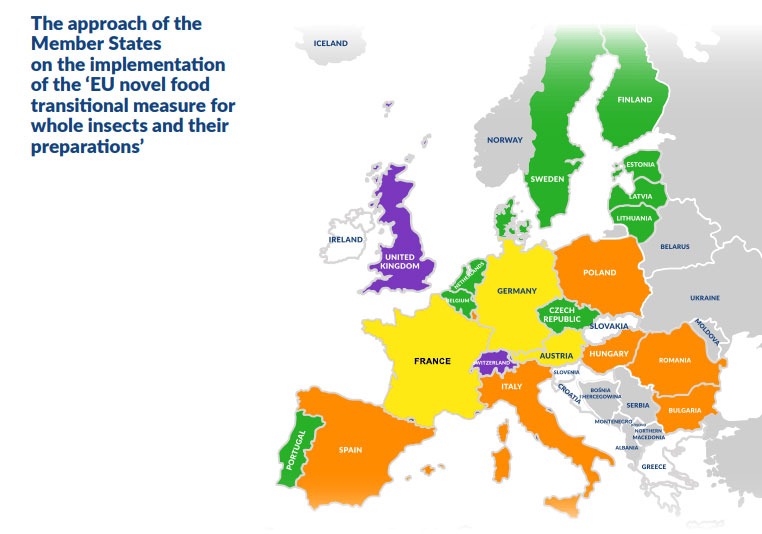
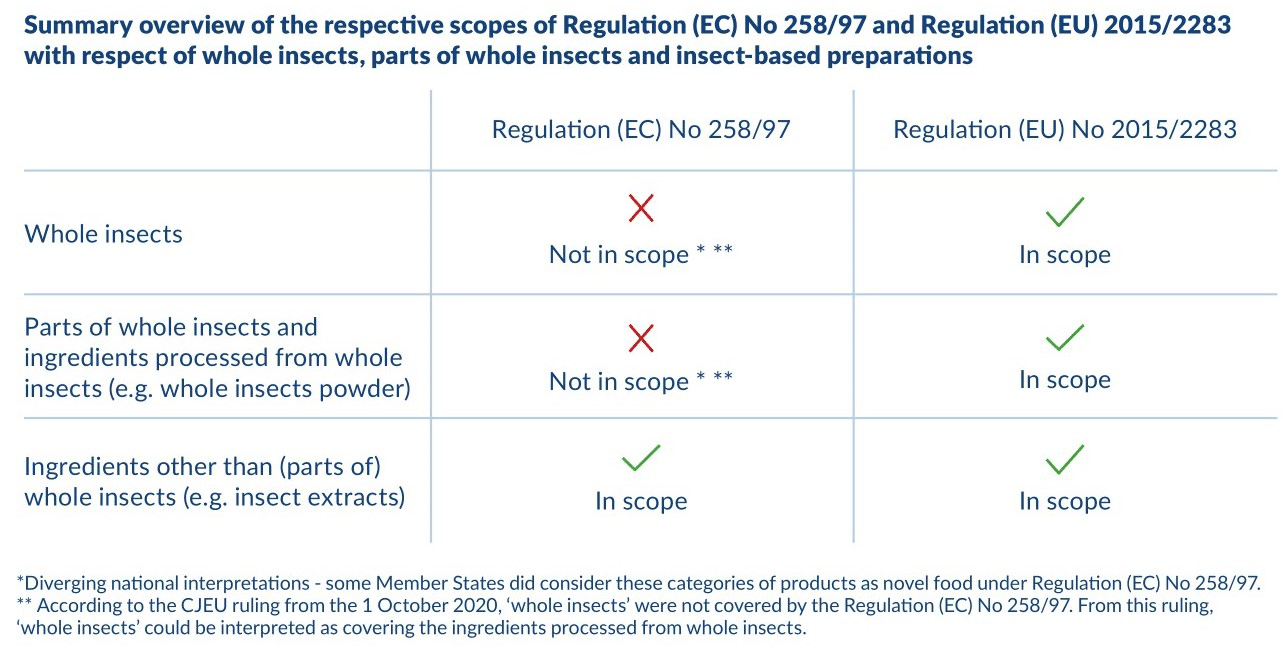 Summary overview of the respective scopes of Regulation (EC) No 258/97 and Regulation (EU) 2015/2283 in respect of whole insects, parts of whole insects and insect-based preparations
Summary overview of the respective scopes of Regulation (EC) No 258/97 and Regulation (EU) 2015/2283 in respect of whole insects, parts of whole insects and insect-based preparations